《Nano Lett.》: Selective CO2 Photoreduction into CH4 Triggered by Metal-Vacancy Pair Sites
Jiacong Wu,+ Juncheng Zhu,+ Wenya Fan,+ Dongpo He, Qinyuan Hu, Shan Zhu, Wensheng Yan, Jun Hu, Junfa Zhu, Qingxia Chen,* Xingchen Jiao,* and Yi Xie*
Selectively achieving carbon dioxide (CO2) photoreduction into methane (CH4) remains significant challenges, which primarily arises from the complexity of the protonation process. Herein, we designed the metal-vacancy pair sites in defective metal-oxide semiconductors, which anchor the reactive intermediates with a bridged linkage for the selective protonation to produce CH4. As an example, the oxygen-deficient Nb2O5 nanosheets are synthesized, in which the niobium-oxygen vacancy pair sites are demonstrated by the X-ray photoelectron spectroscopy and the electron paramagnetic resonance spectra. In situ Fourier transform infrared spectroscopy monitors the *CH3O intermediate, a key intermediate for CH4 production, during the CO2 photoreduction in the oxygen-deficient Nb2O5 nanosheets. Importantly, the built metal-vacancy pair sites regulate the *CH3O formation step as a spontaneous process, making CO2 reduction to CH4 the preferred method. Therefore, the oxygen-deficient Nb2O5 nanosheets exhibit CH4 formation rate of 19.14 μmol g−1 h−1, with the electron selectivity up to 94.1%.
Link: https://pubs.acs.org/doi/10.1021/acs.nanolett.3c04012
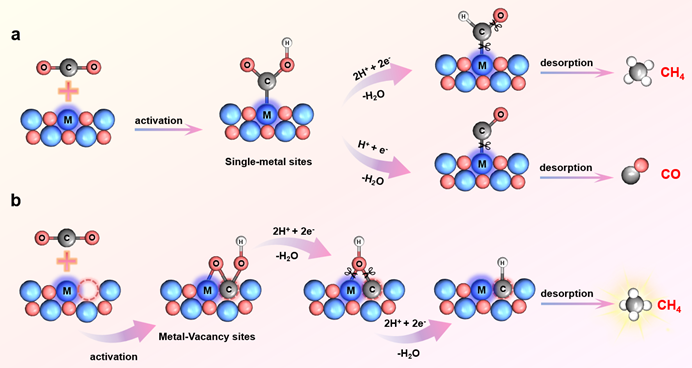
Figure 1. Schematic illustration for tailoring the reaction step in CO2 reduction pathways over the catalyst with (a) the single-metal sites and (b) the metal-vacancy pair sites.