《Adv. Energy Mater.》: Induced C−C Coupling by Amorphous-Crystalline Hybrid Structure for Selective CO2 Photoreduction into C2 Fuels
Dongpo He,+ Guangbing Huang,+ Jun Hu,+ Jinyu Ding, Wenxiu Liu, Liang Chen*, Wensheng Yan, Junfa Zhu, Shan Zhu, Qingxia Chen*, Xingchen Jiao*, and Yi Xie
Selective photoreduction of carbon dioxide (CO2) into high-value C2 products remains a formidable challenge due to the elusive C−C coupling step. Herein, we first introduce the novel concept that an amorphous-crystalline hybrid structure can galvanize previously inert metal atoms, thereby establishing highly active dual sites. This ingenious configuration promotes the C−C coupling, paving the way for CO2 photoreduction into C2 products. Taking the Bi2MoO6 nanosheets anchored by amorphous FeOOH species as an example, X-ray photoelectron spectroscopy (XPS) spectra and X-ray absorption near edge structure (XANES) spectra and density functional theoretical (DFT) calculations confirm the electron transfer from FeOOH to Bi2MoO6 nanosheets. Thus, the introduction of FeOOH activates the nonoperative Bi sites for the construction of Bi−Mo dual sites, verified by the in situ XPS spectra and DFT calculations. Gibbs free energy calculations revealed the formation energy barrier of C−C coupling was hugely lowed from 3.41 to 0.45 eV thanks to the presence of FeOOH species. Therefore, the FeOOH-Bi2MoO6 nanosheets are a game changer, delivering the sole liquid product, acetic acid, with an impressive electron selectivity of approximately 86.9%. In contrast, the Bi2MoO6 nanosheets lag behind, only capable of producing carbon monoxide from CO2 photoreduction.
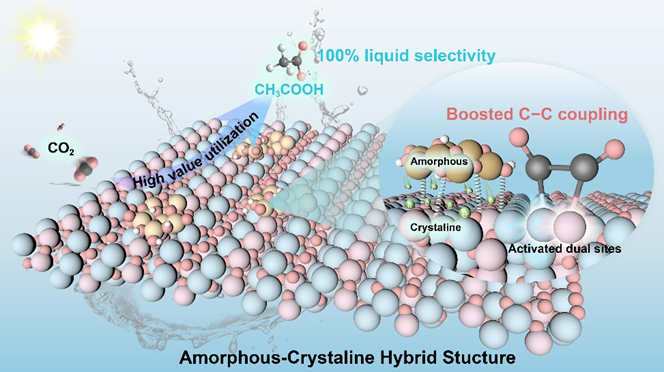
Figure 1. Schematic illustration for CO2 photoreduction into CH3COOH over the catalyst with amorphous-crystalline hybrid structure.