《Angew. Chem. Int. Ed.》: Interface-Engineering-Induced C–C Coupling for C2H4 Photosynthesis from Atmospheric-Concentration CO2 Reduction
Peijin Du,+ Jinyu Ding,+ Chengyuan Liu,+ Peipei Li, Wenxiu Liu, Wensheng Yan, Yang Pan, Jun Hu, Junfa Zhu, Xiaodong Li,* Qingxia Chen,* Xingchen Jiao*
Producing ethylene (C2H4) from carbon dioxide (CO2) photoreduction under mild conditions is primarily restricted by the difficulty of C−C coupling. Herein, we designed highly active metal atom clusters anchored on semiconductor nanosheet, which established heteroatom sites on the interface to steer C−C coupling, realizing air-concentration CO2 photoreduction into C2H4 in pure water for the first time. As an example, the Pd nanoclusters loaded on ZnO nanosheets are prepared, demonstrated by the X-ray photoelectron spectroscopy and high-angle annular dark-field image. In situ Fourier transform infrared spectroscopy confirms the C−C coupling step over the Pd-ZnO nanosheets, while quasi in situ X-ray photoelectron spectroscopy illustrates the active sites of Pd and Zn atoms on the Pd-ZnO nanosheets during CO2 photoreduction. Density functional theoretical calculations unveil the transition state energy barrier of C–C coupling of CO* and COH* intermediates are only 0.998 eV, hinting the easy C–C coupling to produce C2 fuels. Therefore, the Pd-ZnO nanosheets first realize C2H4 photosynthesis by atmospheric-concentration CO2 reduction with the formation rate of 1.03 μmol g−1 h−1, while the ZnO nanosheets only acquired the carbon monoxide product.
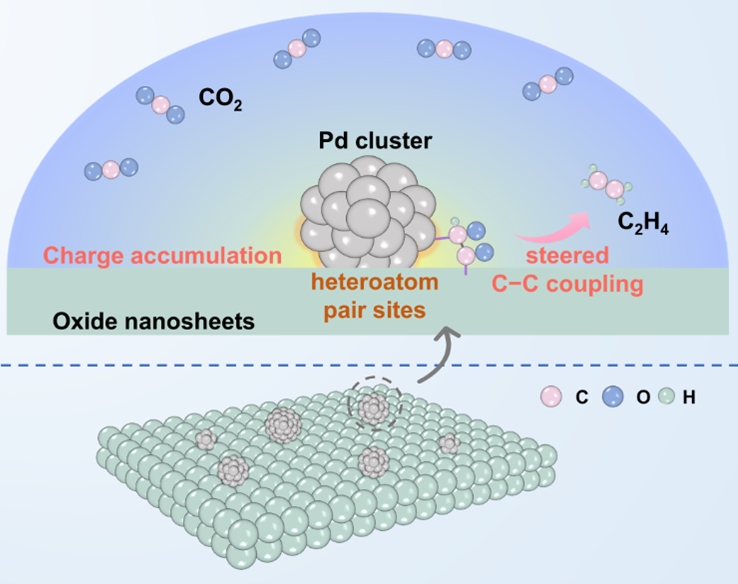
Figure 1. Schematic illustration for CO2 photoreduction over highly active metal clusters on a semiconductor nanosheets, where the constructed heteroatom pair sites with different charge densities on the interface can steer C−C coupling to produce C2H4.