《Nano Lett.》: Infrared-light-driven CO2 Reduction Realized by Charge-Asymmetrical Metallic Conductor
Qinyuan Hu,+ Zhixing Zhang,+ Yanglu Yu,+ Wenxiu Liu, Jiaqi Xu, Wensheng Yan, Jun Hu, Junfa Zhu, Yang Pan, Jianrong Zeng, Xiaodong Li,* Qingxia Chen,* Xingchen Jiao,* and Yi Xie
Until now, there has been a paradox in the utilization of infrared (IR) light, which carries a significant amount of solar energy (around 50% of the spectrum), for carbon dioxide (CO2) photoreduction. Given this, we propose a metallic conductor with charge-asymmetrical active sites, which realize IR-driven CO2 reduction into C2 fuels using water as the reducing agent. Taking the CuInS2 nanosheets as an example, their metallic nature is verified by the valence-band X-ray photoelectron spectroscopy and theoretical calculations, which enables the IR light absorption. Their charge-asymmetrical active sites, confirmed by Bader charge calculations, promotes the C−C coupling. We employ the cobalt atom doping to increase the asymmetric charge distribution on the Cu and In atoms in the CuInS2 nanosheets, lowering the *COH−CO formation energy barrier. These results further verify the charge-asymmetrical active sites in metallic conductor can boost C−C coupling for generating C2 products for IR-driven CO2 reduction.
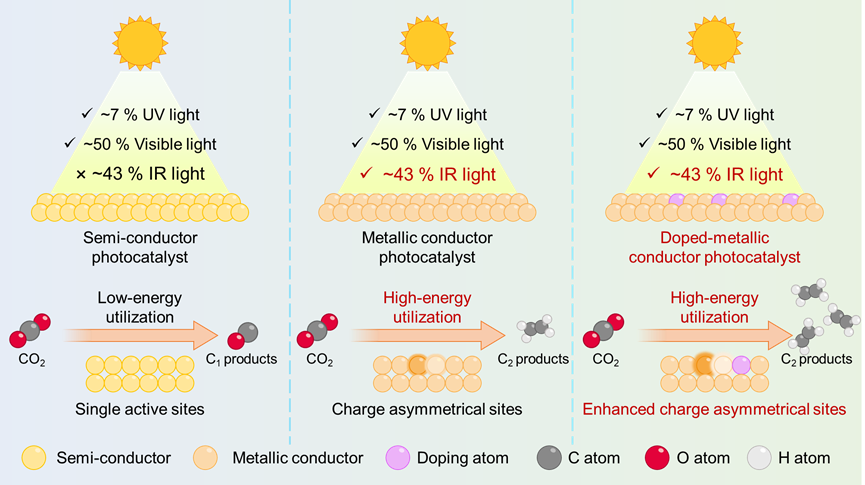
Figure 1. Schematic illustration for IR-driven CO2 reduction into C2 product over the conductor catalyst with charge-asymmetrical dual active sites, in which the conductor catalyst enables the IR light absorption and the charge-asymmetrical dual active sites promote the C–C coupling.