《Angew. Chem. Int. Ed.》: Selective CO2-to-C2H4 Photoconversion Enabled by Oxygen-Mediated Triatomic Sites in Partially Oxidized Bimetallic Sulfide
Yang Wu,+ Qingxia Chen,+ Juncheng Zhu,+ Kai Zheng, Mingyu Wu, Minghui Fan, Wensheng Yan, Jun Hu, Junfa Zhu, Yang Pan, Xingchen Jiao,* Yongfu Sun,* and Yi Xie
In the past few decades, the massive use of fossil energy has led to excessive emissions of carbon dioxide ( CO2 ), causing serious climate and environmental problems. Interestingly, the conversion of CO2 into various types of carbon-based fuels through renewable solar energy is one of the most promising solutions, which not only helps to solve the problem of environmental degradation, but also alleviates the energy crisis. Compared with C1 product, C2 product has higher energy density and market value. However, due to the huge kinetic challenges of C-C coupling, selective photoreduction of CO2 to C2 fuel under mild conditions still has problems such as low yield and poor selectivity. Therefore, it is very important to develop highly active catalysts for photoreduction of CO2 to C2 products.
In view of this, the partially oxidized bimetallic sulfides designed by the author achieved highly selective photoreduction of CO2 to C2 products by promoting the C−C coupling process. They were the first to synthesize FeCoS2 ultrathin sheets with different degrees of oxidation. DFT theoretical calculations confirmed that the original Co-Fe double sites were changed into Co-O-Fe triple sites by introducing oxygen atoms, and abundant charges were accumulated around the oxygen atoms, which was beneficial to promote the C-C coupling of the two *COOH intermediates. Therefore, the moderately oxidized FeCoS2 ultrathin sheets have high-efficiency photoreduction of CO2 to C2H4. The formation rate of C2H4 is 20.1 μmol g−1 h−1, and the product selectivity and electron selectivity are 82.9 % and 96.7 %, respectively, which is superior to most photocatalysts previously reported under similar conditions.
In order to further study the effect of partially oxidized FeCoS2 ultrathin sheets on the C-C coupling process, the author used in-situ characterization techniques and density functional theory calculations to explain the mechanism of photoreduction of CO2 to C2H4. In the perfect FeCoS2 ultrathin nanosheets, the Co-Fe biatomic sites and HOOC-COOH* intermediates are extremely unstable, while the Co-O-Fe triatomic sites formed in the partially oxidized FeCoS2 ultrathin nanosheets can be closely combined with the HOOC-COOH* intermediates. In other words, after the partial oxidation process, the Co-Fe double site becomes the Co-O-Fe triple atom site, so that the HOOC-COOH* intermediate can be tightly adsorbed on the partially oxidized FeCoS2 ultrathin sheet, stabilizing the intermediate of the reaction process. In addition, the differential charge density calculation shows that the charge tends to accumulate around the introduced oxygen atoms, resulting in a higher charge density of the C-O bond formed between the partially oxidized FeCoS2 ultrathin sheet and the HOOC-COOH* intermediate. This also proves that the partially oxidized FeCoS2 ultrathin sheet is easier to achieve C-C coupling. In this paper, it is experimentally / theoretically verified that the triatomic sites constructed by partial oxidation can promote C-C coupling and improve the selectivity and activity of photoreduction of CO2 to C2H4, which provides a new way to obtain efficient photoreduction of CO2 to C2 products. This work was published in the internationally renowned journal ' Angewandte Chemie International Edition .
The first author of this paper is Associate Prof. Qingxia Chen of Jiangnan University, Dr. Yang Wu and Dr. junchen zhu of University of Science and Technology of China. The corresponding authors are Prof. Yongfu Sun of University of Science and Technology of China and Prof. Xingchen Jiao of Jiangnan University.
Link: https://doi.org/10.1002/anie.202301075
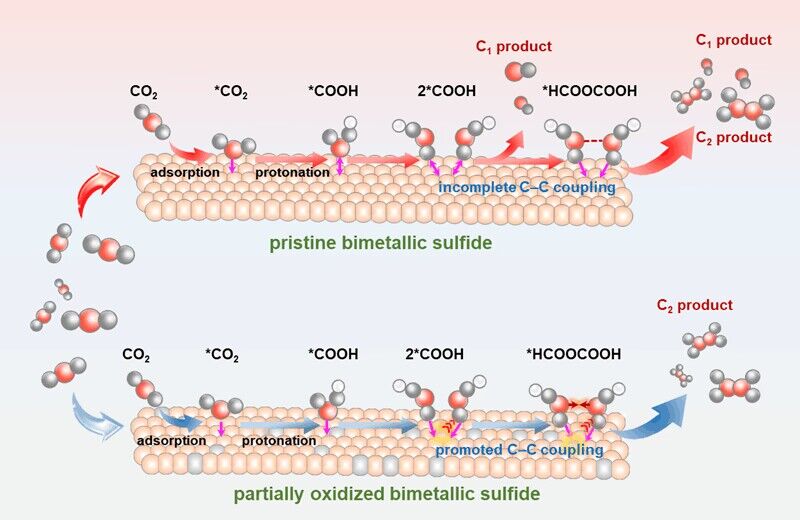
Figure 1. Schematic illustration for modulating the C-C coupling step in CO2 reduction pathways over the pristine bimetallic sulfide and the partially oxidized bimetallic sulfide.