《ACS Catal.》: Constructing Charge-Asymmetrical Au Pair Sites for Photoreduction of Air-Concentration CO2 into CH3COOH
Qinyuan Hu,+ Jiawei Xie,+ Fangming Zhao,+ Zhixing Zhang, Yanglu Yu, Xiaodong Li,* Wensheng Yan, Jun Hu, Junfa Zhu, Yang Pan, Meng Zhou,* Yuming Dong,* and Xingchen Jiao*
The photoreduction of carbon dioxide (CO2) into C2 products faces a significant C–C coupling challenge. Charge asymmetry active sites constructed through loading engineering can effectively promote C–C coupling. Herein, we propose that the Au atom pairs with an asymmetric charge distribution in the ZnNb2O6 nanosheets trigger the acetic acid (CH3COOH) formation for photoreduction of air-concentration CO2. Density functional theory calculations indicate that the Au-ZnNb2O6 nanosheet slab has the potential to tailor CO2-to-CH3COOH pathway own to the presence of Au atom pairs. In situ Fourier transform infrared spectra detect the *COCOH intermediate, confirming the occurrence of C–C coupling, while quasi in situ X-ray photoelectron spectroscopy verifies the Au atoms functioned as the active sites. The TA spectra revealed the electron transfer process from ZnNb2O6 nanosheets to Au particles. Accordingly, the Au-ZnNb2O6 nanosheets achieve a CH3COOH formation rate of 2.19 μmol g–1 h–1 and a selectivity of 50.5%, whereas pristine ZnNb2O6 nanosheets produce only CO products.
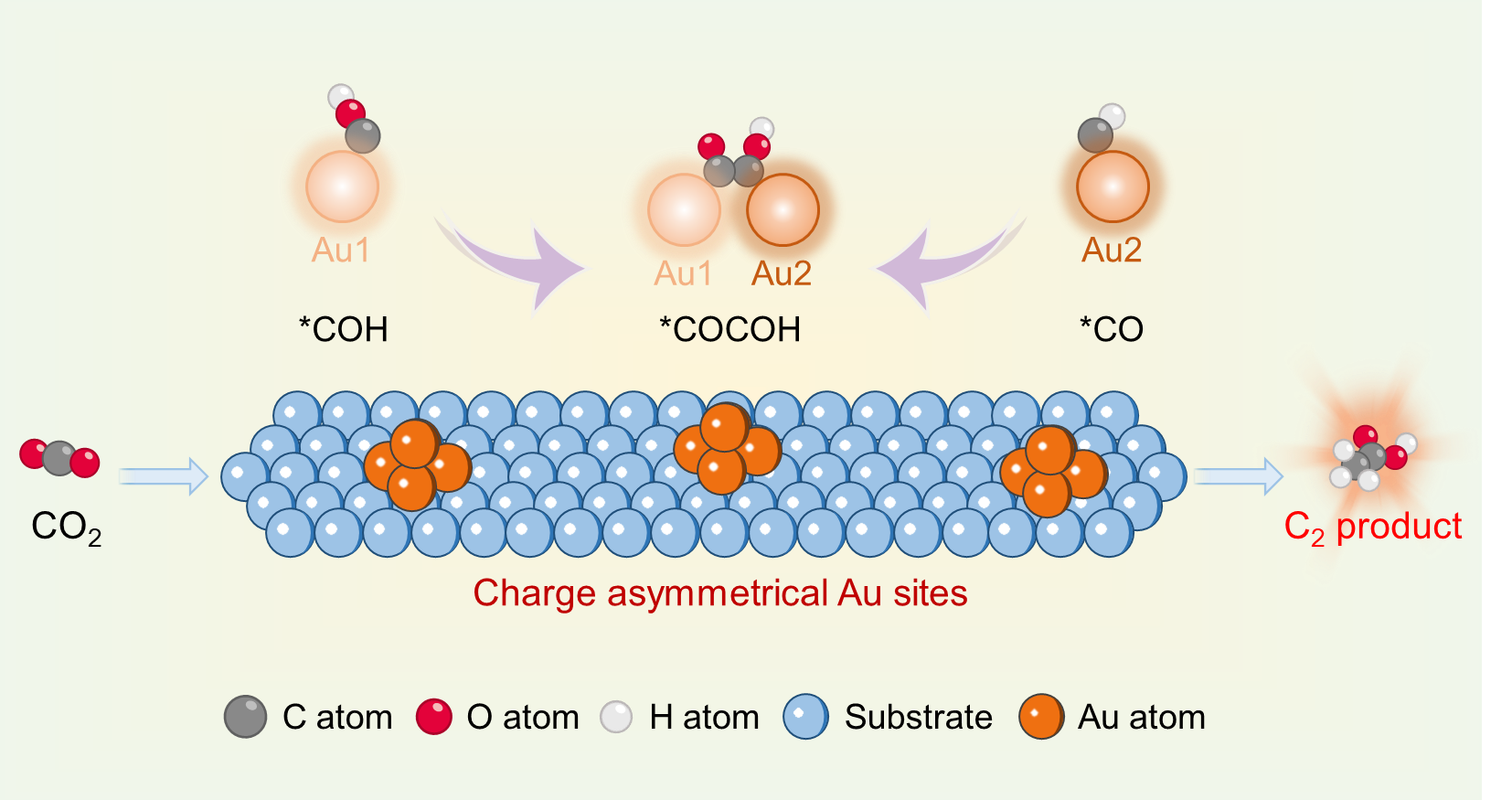
Figure 1. Schematic illustration for photoreduction of CO2 into C2 products over the charge asymmetrical Au sites.